The EU and AstraZeneca have reached an agreement which will secure the delivery of the remaining COVID-19 vaccine doses to Member States under the terms of the Advance Purchase Agreement concluded on 27 August 2020 with AstraZeneca. The agreement will also end the pending litigation before the Brussels Court. Commissioner for Health and Food Safety […]
Read MoreSugars, salt, fat, fibre: are packaged foods and soft drinks becoming healthier?
A recent JRC study analysed trends in the nutritional quality of the packaged food and non-alcoholic beverage by assessing the level of sugars, salt, saturated fat and fibre of these products sold in supermarkets across Europe. All EU Member States provide dietary recommendations for a healthy diet to their citizens. The foods offered in supermarkets […]
Read MoreCommission approves new contract for a potential COVID-19 vaccine with Novavax
The European Commission has approved its seventh Advanced Purchase Agreement (APA) with a pharmaceutical company to ensure access to a potential vaccine against COVID-19 in Q4 of 2021 and in 2022. Under this contract, Member States will be able to purchase up to 100 million doses of the Novavax vaccine, with an option for 100 […]
Read MoreHow often do you drink sugar-sweetened soft drinks?
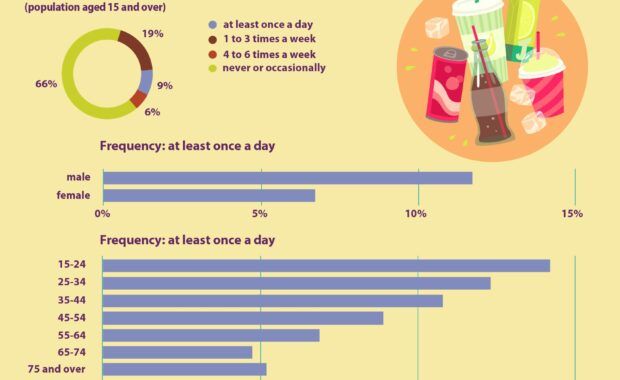
In 2019, 9% of people aged 15 and over in the EU drank sugar-sweetened soft drinks daily, while 6% drank such beverages 4-6 times a week and 19% drank these 1-3 times a week. Daily consumption of sugar-sweetened soft drinks was more common for men than for women (12% of men vs. 7% of women). In addition, […]
Read MoreSafety of dietary sugars: draft opinion open for public consultation
EFSA’s expert panel on nutrition has completed its provisional assessment of the safety of dietary sugars following a comprehensive scientific review. Valeriu Curtui, head of EFSA’s Nutrition Unit, said: “This has been a hugely challenging work so far, involving the evaluation of over 30,000 publications. Our experts and staff have made an immense effort to reach this […]
Read MoreOver half of adults in the EU are overweight
While 45% of adults living in the EU had a normal weight in 2019, slightly more than half (53%) were considered as overweight (36% pre-obese and 17% obese) and almost 3% as underweight, according to their body mass index (BMI). BMI is a measure of a person’s weight relative to their height that links fairly well with body fat. With the exception of […]
Read MoreCOVID-19 evolution in Europe – 1st half 2021
Through data published by Johns Hopkins University and collected daily by the European Documentation Center in Almeria, an analysis of the evolution of the COVID-19 pandemic in Europe during the first half of the year 2021 has been performed. During the first half of 2021, COVID-19 cases continued to increase steadily. The European continent began […]
Read MoreVaccination in Europe – First semester 2021
Although the vaccination process is progressing steadily in all EU countries, the pace of vaccination is uneven in the different European territories, with some countries being more efficient in vaccinating their population. As of July 30, 39.21% of the EU/EEA population had received the full course of one of the EU-licensed vaccines and had therefore […]
Read MoreCOVID-19 developments in Europe – 1st quarter 2021
During the first quarter of 2021, COVID-19 cases continued to increase steadily. The European continent started the year with a total of 27,552,498 people infected with COVID-19 and 585,110 deaths from COVID-19. As of 31 March, the number of reported cases in Europe grew by 63.74% to 45,115,099 positive cases. Unfortunately, deaths also rose by […]
Read More