Around 1,000 detections of highly pathogenic avian influenza (HPAI) were reported in 25 EU/EEA countries and the UK between 8 December 2020 and 23 February 2021, according to the latest overview of HPAI in Europe. The majority of the 1,022 detections – 592 – were reported in domestic poultry, with wild and captive birds making up […]
Read MoreSARS-CoV-2 in mink: recommendations to improve monitoring
Early detection of SARS-CoV-2 (coronavirus) should be a priority objective for monitoring activities at mink farms in the European Union, a new report recommends. The report, compiled by EFSA and the European Centre for Disease Prevention and Control (ECDC), proposes options for monitoring strategies that will help to prevent and control spread of the disease. It concludes […]
Read MoreEMA receives application for conditional marketing authorisation of COVID-19 Vaccine Janssen
EMA has received an application for conditional marketing authorisation (CMA) for a COVID-19 vaccine developed by Janssen-Cilag International N.V. EMA’s human medicines committee (CHMP) will assess the vaccine, known as COVID-19 Vaccine Janssen, under an accelerated timetable. The Committee could issue an opinion by the middle of March 2021, provided the company’s data on the vaccine’s efficacy, safety […]
Read MoreHERA Incubator to anticipate the threat of COVID-19 variants
Von der Leyen announces the start of HERA Incubator to anticipate the threat of coronavirus variants To prepare Europe for an increased threat of coronavirus variants, the President of the European Commission Ursula von der Leyen announced the start of European bio-defence preparedness plan called “HERA Incubator”. The Health Emergency Preparedness and Response Authority (HERA) Incubator will […]
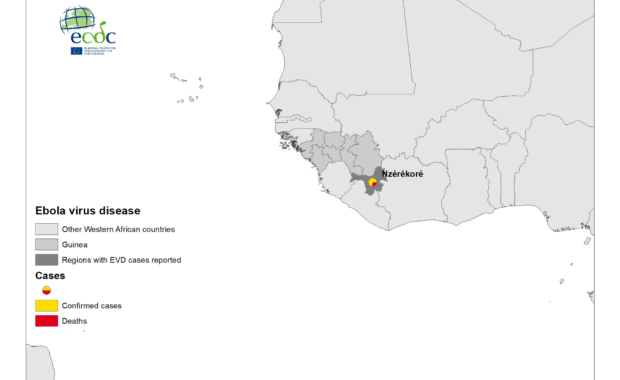
Read MoreOutbreak of Ebola virus disease in Africa
As of 14 February 2021, four cases of Ebola virus disease (EVD), including two deaths, have been reported in the North Kivu province in the eastern part of the Democratic Republic of the Congo (DRC) as well as seven cases in Guinea, including three deaths. On 7 February 2021, the Minister of Health of the […]
Read MoreEMA preparing guidance to tackle COVID-19 variants
EMA is developing guidance for manufacturers planning changes to the existing COVID-19 vaccines to tackle the new virus variants. In order to consider options for additional testing and development of vaccines that are effective against new virus mutations, the Agency has requested all vaccine developers to investigate if their vaccine can offer protection against any […]
Read MoreCOVID-19 Evolution – 2020
This document collects and analyzes the data on the evolution of COVID-19, collected by the Johns Hopkins University and published daily by the European Documentation Center of Almeria during the year 2020. On January 24, 2020 France reported the first case of COVID-19, being the first case, being the first known case of COVID-19 in […]
Read MoreEU urges AstraZeneca to explain vaccine delivery delays
▶️ Watch the press conference by Commissioner @SKyriakidesEU on vaccine deliveries and on the export transparency scheme. #StrongerTogether https://t.co/iczvKiAiRm — European Commission 🇪🇺 (@EU_Commission) January 25, 2021 Last Friday, the company AstraZeneca surprisingly informed the Commission and the European Union Member States that it intends to supply considerably fewer doses in the coming weeks than […]
Read MoreCommission concludes exploratory talks with Valneva to secure a new potential vaccine
The European Commission concluded exploratory talks with the pharmaceutical company Valneva with a view to purchasing its potential vaccine against COVID-19. The envisaged contract with Valneva would provide for the possibility for all EU Member States to purchase together 30 million doses, and they could further purchase up to 30 million more doses. This finalisation […]
Read More