The system developed by ADmit Therapeutics is expected to reach the market later this year and will be a giant step in the fight against Alzheimer’s.
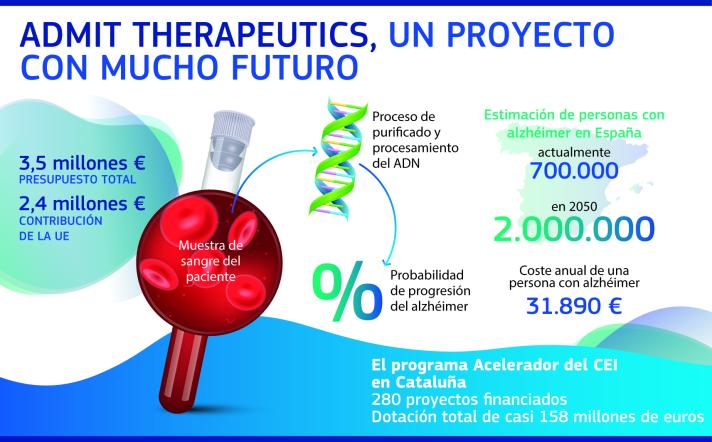
“We have developed an algorithm that allows to determine the percentage of probability of development of Alzheimer’s”
The system developed by ADmit Therapeutics is expected to reach the market later this year and will be a giant step in the fight against Alzheimer’s. On the one hand, it offers the affected person a very relevant information. On the other hand, it will be key to find a treatment that cures the disease, being able to select the right patients for clinical trials.
A viable project thanks to EU funding
The development of this test has been possible thanks to the support of Europe, more specifically, the CIS Accelerator programme. “It is thanks to the European Union that we are where we are. Without the support of the EU it would not have been possible to move forward”, emphasises Barrachina, adding: getting help is a complex and very competitive process, but very rewarding, as you involve the whole team and learn from everyone.
- Marta, how did the team live the news of achieving European funding for the project?
“It was an indescribable, enormous joy. We were at a turning point. To continue advancing we needed 4 million euros and our project was high risk. We were in a very early phase and there is a lot of fear around everything Alzheimer’s about the rate of failures in these investments. In addition, we have developed a diagnostic tool and betting on it without an existing cure… it was complicated. Our project was the kind that needed these supports”.
- In what way does it affect, therefore, its development?
“It allowed us to grow and professionalise: we go from being an entrepreneurial team to a professional team, with international projection, already starting to have the profile of industrial team, with the seal of quality offered by the European Union”.
How do innovation projects like this benefit society?
“We are the first to understand that it is a risk to invest in this type of projects, but if you end up succeeding, everything that society has invested in you, you return it multiplied by many digits”.
- Four tips for any company that intends to qualify for this aid
- Write the application for funding with the whole team: from the technological area to the commercial.
- Have a rigorous and coherent technical-scientific foundation.
- Be resilient. We got it the first time, but it’s not frequent.
- Integrate the comments of the evaluators if the request does not go to the first.
For further information: European Commission





Leave a Reply