Clinical trials of a new prototype vaccine designed to selectively target and eradicate the early spread of cancer cells represent another step in the fight against cancer.
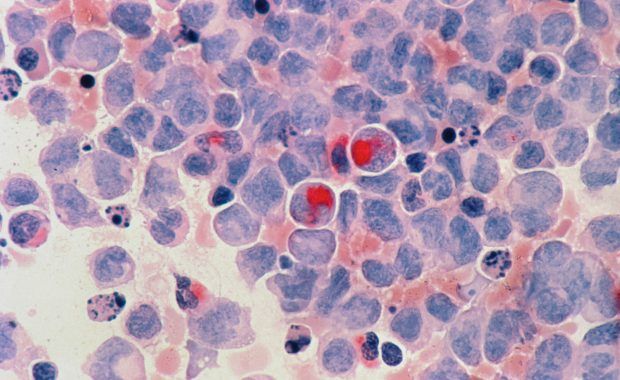
While most cancer treatments focus on treating the primary tumour, the leading cause of cancer death is metastasis, the spread of cancer cells from the primary tumour to surrounding tissues and organs. However, efforts to reduce metastatic growth have so far been unsuccessful. This fact leads to a high recurrence rate, up to 40% in prostate cancer. “In this group of patients, 90% eventually died of metastatic disease,” says Alexandra Ellervik, coordinator of the RV001 project at RhoVac (Denmark). “The discovery of a treatment method that is effective against the formation of early metastases will be a turning point in the treatment of prostate cancer.
Development of vaccines against metastatic cells
The EU-funded RV001 project aims to promote promising developments in this field. Danish biotech company RhoVac has developed a prototype vaccine against metastatic cancer cells to significantly reduce cancer recurrences. The immunotherapy, called RV001, activates the patient’s immune system to recognise and destroy metastatic cancer cells. “The immune system is activated by exposure to the RhoC antigen,” explains Ellervik. By activating the immune system, it is trained to find and destroy cancer cells that overexpress RhoC, a protein associated with metastatic cells. “By activating T cells to selectively target only cells that overexpress RhoC, the body’s natural defence system leaves healthy, normal cells untouched. “Our idea is that RV001 can be administered immediately after the primary tumour is removed, thus preventing metastatic cancer cells from forming and spreading to other organs and tissues,” Ellerwick said.
February 4: World Cancer Day |
Running prototype vaccine in clinical trials
The EU-funded RV001 project sought to build on promising preclinical evidence. For this, the project enabled ambitious clinical trials to be conducted across five European countries, involving a total of 180 patients. “Our ambition was to vaccinate against metastatic cancer. This is what we were trying to prove, and was the great unmet medical challenge we hoped to successfully address.” Unfortunately, the project team was unable to record the clinical results they were looking for. Even though the expected immune response did materialise, the clinical trial did not reach its end points. No patient experienced any severe adverse effects during the trial.
New avenues to treating cancer
For Ellervik, this project reflects the reality of clinical trials. “The success rate for oncological trials at this phase II stage is around 50 %,” he adds. “Even at the following clinical phase III, the success rate is only 70 %.” Furthermore, Ellervik believes that EU funding not only provided essential financial support, but also provided a quality stamp for the project, the company and the shareholders who had financed the lion’s share of the trial. There are also valuable lessons that can be taken away. “Perhaps in the future a similar treatment can be developed that combines other immune oncology therapies, and delivers the positive treatment effects we were looking for.” Ultimately therefore, the RV001 trials represent another necessary step towards identifying and bringing effective cancer therapies to market. “This project was one small piece in a much bigger puzzle,” says Ellervik.





Leave a Reply