EMA’s safety committee (PRAC) is reviewing very rare cases of unusual blood clots that occurred in the United States following the use of Janssen’s COVID-19 vaccine. The type of blood clot reported, cerebral venous sinus thrombosis (CVST), occurred in most cases in combination with low levels of blood platelets (thrombocytopenia).
The US FDA and CDC recommended that the use of the vaccine should be paused while they review six reported cases in the United States. More than 6.8 million doses of the vaccine have been administered.
Janssen has announced their decision to proactively delay the rollout of the vaccine in the EU while investigations continue. The vaccine was authorised in the EU on 11 March 2021 but widespread use of the vaccine within the EU has not yet started. The company is in contact with national authorities, recommending to store the doses already received until the PRAC issues an expedited recommendation.
EMA is investigating all the cases reported and will decide whether regulatory action is necessary. The Agency is working closely with the US FDA and other international regulators.
EMA is expediting this evaluation and currently expects to issue a recommendation next week. While its review is ongoing, EMA remains of the view that the benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects. The Agency’s scientific opinions provide EU Member States with the information they need to take decisions on the use of vaccines in their national vaccination campaigns.
More about the vaccine
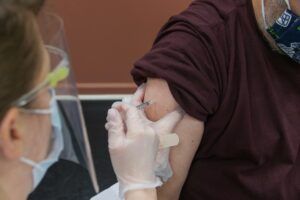
COVID-19 Vaccine Janssen is a vaccine for preventing coronavirus disease 2019 (COVID-19) in people aged 18 years and older. It is made up of another virus (of the adenovirus family) that has been modified to contain the gene for making a protein found on SARS-CoV-2.
The most common side effects with COVID-19 Vaccine Janssen are usually mild or moderate and get better within 1 or 2 days after vaccination.
More about the procedure
The review is being carried out in the context of a safety signal, under an accelerated timetable. A safety signal is information on a new or incompletely documented adverse event that is potentially caused by a medicine such as a vaccine and that warrants further investigation. The presence of a safety signal does not necessarily mean that a medicine has caused the reported adverse event. The assessment of safety signals seeks to establish whether a causal relationship between the medicine and the adverse event is at least a reasonable possibility.
The review is being carried out by EMA’s Pharmacovigilance Risk Assessment Committee (PRAC), the committee responsible for the evaluation of safety issues for human medicines. Once the review is completed, PRAC will make any recommendations necessary to minimise risks and protect people’s health.


Leave a Reply