The Council agreed its position (‘general approach’) on the revision of the consumer credit directive. The revised directive repeals and replaces the current 2008 directive on credit agreements. Since 2008, the increasing digitalisation of the European economy has led to significant changes in the consumer credit market. This has led to the emergence of new […]
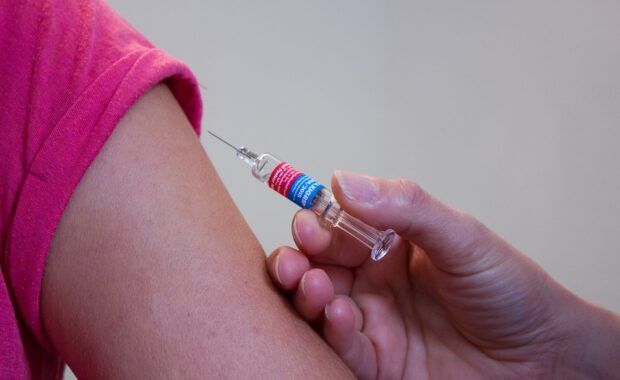
Agreement with Moderna to adapt delivery schedules
The European Commission and the vaccine developer Moderna have reached an agreement to ensure that the delivery of COVID-19 vaccines is adapted to the needs of Member States. On the basis of this agreement, the company will postpone the delivery of some doses initially planned for quarter 2 of 2022, to later in the year. In […]
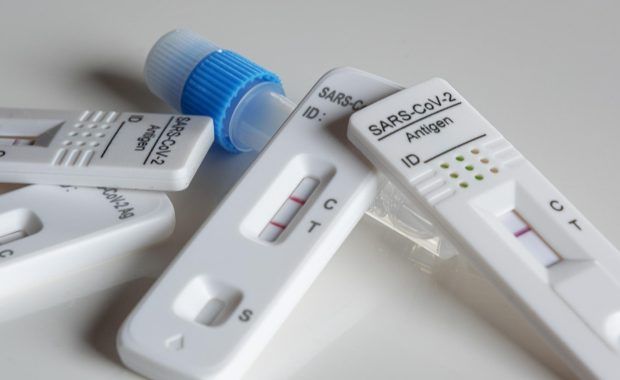
Stronger rules for placing medical tests on the market
As of tomorrow, new rules on in vitro diagnostic medical devices (IVDR) such as HIV tests, pregnancy tests or COVID-19 tests, will be applicable. The rules will better protect public health and patient safety in respect to these devices, bringing EU law in line with technological advances and progress in medical science. By aligning market […]
New agreement to recognise COVID-19 as an occupational disease
On 18th May, Member States, workers and employers in the EU Advisory Committee on Safety and Health at Work (ACSH) reached an agreement on the need to recognise COVID-19 as an occupational disease in health and social care and in domiciliary assistance and, in a pandemic context, in sectors where there is an outbreak in […]
Indonesia is connected to the EU Digital COVID Certificate system
Good news for Indonesian and European travellers: EU and Indonesia have agreed the mutual recognition of their COVID vaccine certificates. This means that, when entering the EU or travelling between EU Member States, holders of the Indonesian vaccine certificate can use it under the same conditions as people holding the EU Digital COVID Certificate (EUDCC). […]
Commission will phase out State aid COVID Temporary Framework
The European Commission will phase out the State aid COVID Temporary Framework, adopted on 19 March 2020 and last amended on 18 November 2021, enabling Member States to remedy a serious disturbance in the economy in the context of the coronavirus pandemic. The State aid COVID Temporary Framework will not be extended beyond the current expiry date, which is 30 […]
Commission steps up funding to vaccination roll-out in Africa
The European Commission has announced its intention to step up funding to accelerate roll-out and uptake of vaccines and other COVID-19 tools in Africa, with a further €400 million in support. The Commission also foresees a €427 million euros ($450 million) contribution to the Global Pandemic Preparedness Fund to support efforts to prevent and better respond to […]
Statement by President von der Leyen ahead of Europe Day
My fellow Europeans, today our continent encounters shadows of a past we thought we had long left behind. An atrocious war, senseless aggression and destroyed cities. Millions of innocents fleeing their homes. A people desperately struggling to determine its own future. Europe stands at the side of Ukraine. At the same time the Kremlin’s […]
EU COVID Certificate: committee backs one-year extension to ensure free movement
On Thursday, the Civil Liberties Committee endorsed proposals to keep the EU Digital COVID Certificate framework in place for another year. To ensure that EU citizens can benefit from their right to free movement regardless of the evolution of the COVID-19 pandemic, the Committee on Civil Liberties, Justice and Home Affairs approved two reports to […]