The Commission welcomes the political agreement reached on 7th March by the European Parliament and Member States on the European Year of Skills. Following the announcement by President Ursula von der Leyen in her 2022 State of the Union address, the Commission submitted its proposal on the European Year of Skills to the co-legislators last October. During the European Year […]
Member States update EU list of non-cooperative jurisdictions for tax purposes
On 14 February 2023, EU Member States agreed on the latest update the EU list of non-cooperative jurisdictions for tax purposes. Following the update, Annex I of the EU list is made up of 16 jurisdictions due to their lack of commitment to improve their tax good governance or due to the lack of progress […]
Commission proposes Action Plan to address challenges
Ahead of the Extraordinary Justice and Home Affairs Council (Home Affairs) of 25 November 2022, the Commission is presenting an EU Action Plan on the Central Mediterranean. Whilst emphasising that structural solutions will only be found through agreement on the full set of asylum and migration reforms currently being negotiated, the Commission is proposing a […]
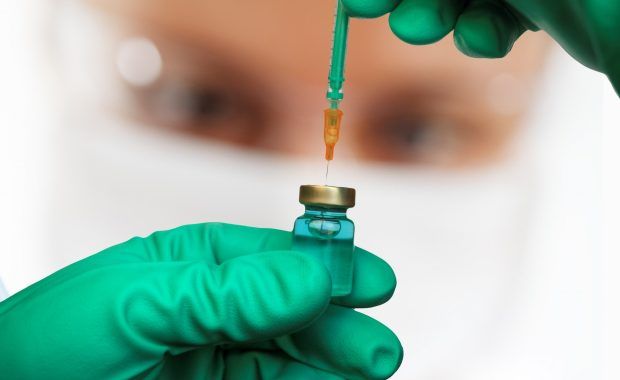
First adapted COVID-19 booster vaccines recommended for approval in the EU
EMA’s human medicines committee (CHMP) has recommended authorising two vaccines adapted to provide broader protection against COVID-19. Comirnaty Original/Omicron BA.1 and Spikevax Bivalent Original/Omicron BA.1 are for use in people aged 12 years and above who have received at least primary vaccination against COVID-19. These vaccines are adapted versions of the original vaccines Comirnaty (Pfizer/BioNTech) […]
Commission urges Member States to transpose EU copyright rules into national law
Last Thursday, the Commission has decided to send reasoned opinions to Bulgaria, Cyprus, Greece, Ireland, Latvia, Poland, Portugal, Slovenia, Slovakia and Finland over their failure to notify the Commission of transposition measures on copyright and related rights applicable to certain online transmissions (EU Directive 2019/789). The Commission has also today sent reasoned opinions to Belgium, […]
Partnerships for Regional Innovation: 63 regions, 7 cities and 4Member States
Yesterday, the Commission announced the 63 regions, seven cities and four Member States selected in the pilot project for Partnerships for Regional Innovation, an initiative developed together with the Committee of the Regions. Participants in the pilot action are open to share good practices and to co-develop and test tools to mobilise multiple sources of […]