Are PFAS the most worrying pollutants of our time, and can we rid ourselves of them? Invisible but ubiquitous, PFAS (perfluoroalkyl and polyfluoroalkyl substances) are found in everything from non-stick pans to smartphones. While these chemicals are essential to modern industry, their “eternal” nature means that they accumulate in our environment and in our bodies, […]
Commission approves up to €403 million in State aid for major EU healthcare project
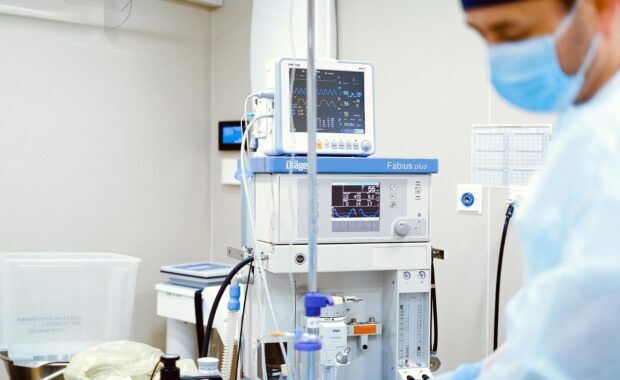
The European Commission has approved, under EU State aid rules, the second health-related Important Project of Common European Interest (‘IPCEI’) to support innovations in medical devices. This includes the introduction of novel digital and artificial intelligence (‘AI’) features in medical devices. The IPCEI will support collaborative research and innovation, as well as the first industrial […]
Commission restricts Chinese participation in medical devices procurement
The European Commission decided to exclude Chinese companies from EU government purchases of medical devices exceeding €5 million. This measure follows the conclusions of the first investigation under the International Procurement Instrument (IPI), and allows no more than 50% of inputs from China for successful bids. This response is proportionate to China’s barriers, while ensuring that all the necessary medical devices […]
The Commission has adopted a proposal to give more time to certify medical devices
The Commission adopted a proposal to give more time to certify medical devices to mitigate the risk of shortages. The proposal introduces a longer transition period to adapt to new rules, as foreseen under the Medical Devices Regulation. The new deadlines depend on the medical devices’ risk class and will ensure continued access to medical […]